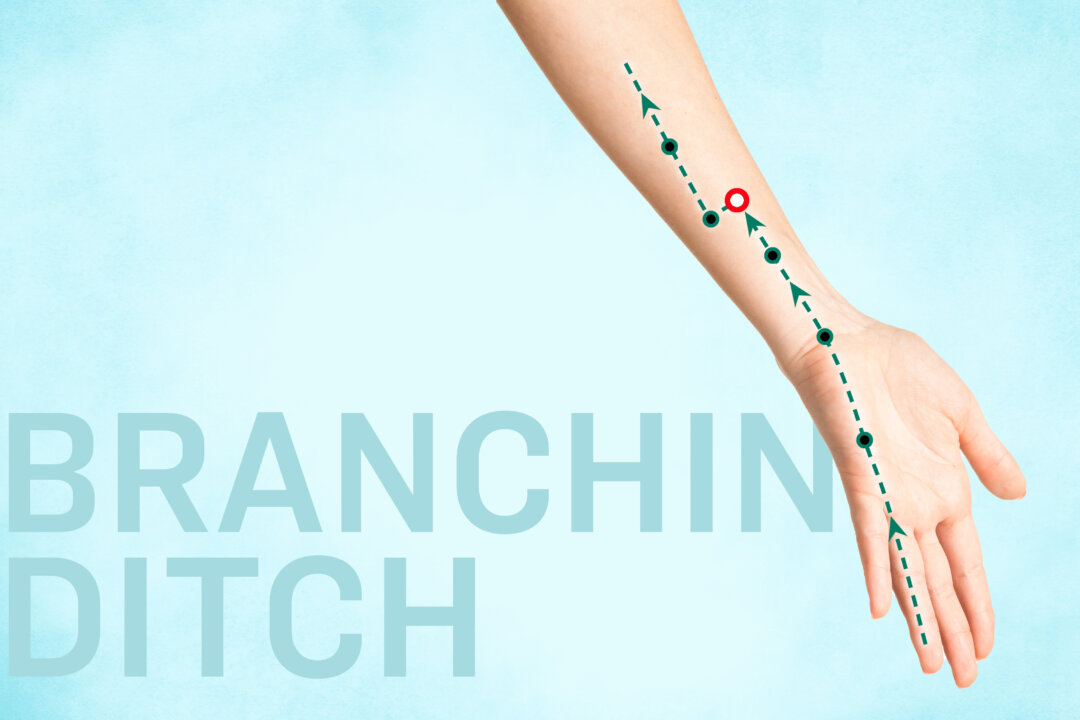
Wave Life Sciences ( NASDAQ: WVE ) announced Wednesday its plans to file a New Drug Application (NDA) with the U.S. FDA in 2026 to seek the regulator’s accelerated approval for WVE-N531, an experimental therapy for a neuromuscular disease known as Duchenne muscular dystrophy.
.