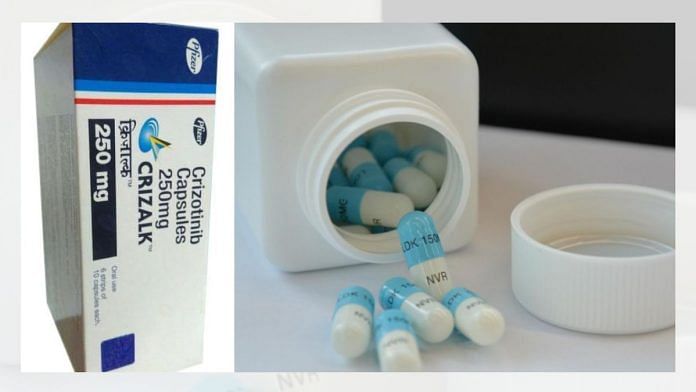
New Delhi: A first-of-its-kind report evaluating the cost-effectiveness of new age targeted therapies for lung cancers—one of the top malignancies in the country which is rising at an alarming rate—has suggested including the innovative medicines in India’s flagship health insurance scheme Ayushman Bharat-PMJAY after price negotiations. The report, commissioned and funded by the Department of Health Research under the Union health ministry, analysed cost-effectiveness of two key precision medicines—crizotinib and ceritinib—comparing them to to standard chemotherapy in patients with newly diagnosed advanced or metastatic non-small cell lung cancer (NSCLC). These targeted therapies are patented, and cost Rs 40,000 to Rs 50,000 a month.
Titled ‘Cost-effectiveness analysis and value-based pricing of anti-cancer drugs in India’ , the report has recommended value-based prices and establishment of institutional structures for strategic purchasing and price negotiations for the new medicines , under the Ayushman Bharat- Pradhan Mantri Jan Aarogya Yojana (AB-PMJAY). ThePrint has seen a copy of the report, prepared by health economists associated with the Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh. Under AB-PMJAY, which includes hospitalisation coverage worth up to Rs 5 lakh, oncology services such as chemotherapy, radiotherapy and surgical oncology are also offered to economically vulnerable families.

According to Globocan, a total of 81,748 cases of lung cancers were detected in India in 2022 —the highest after cancers of breast, lips and oral cavity, and cervix, while malignancy killed 75,031 people. Scientific evidence in recent years has also shown that lung cancer—which has a very poor five-year survival rate at just about 28 percent—is rising alarmingly in India in non-smokers, women and younger age groups. A majority of the patients are developing adenocarcinoma, a more aggressive form of lung cancer.
The health economists’ report estimated that crizotinib, manufactured by US-based pharma giant Pfizer and sold under the brand name Xalkori, and Swiss drugmaker Novartis’s ceritinib, marketed under the brand name Zykadia, offer “higher health gains as compared to chemotherapy”. For these drugs to be a cost-effective option in the Indian context, the prices of ceritinib and crizotinib would need to be cut down drastically, by nearly 92 percent and 81 percent, respectively, the report adds (paraphrased what you had quoted from the report). “Therefore, targeted therapies should be included in India’s publicly financed health insurance scheme at the recommended value-based prices.
” In 2023, the Parliamentary Standing Committee on Health and Family Welfare, in its report ‘Cancer care plan and management, prevention, diagnosis, research and affordability of cancer treatment’, noted that all forms of latest therapies for cancer treatment are not covered under AB-PMJAY, and asked the ministry to explore innovative funding models for inclusion of more targeted oncology treatments under the scheme. “The latest report was an effort to assess the cost-effectiveness in the context of therapeutic advantages offered by new types of drugs to treat cancers and whether it suits our public health financing scheme to consider these drugs in the treatment plan,” a senior health ministry official told ThePrint. A Novartis spokesperson said they were not aware of the report, but added: “We are always willing to work with the government and other stakeholders to find innovative solutions to improve access of our medicines to patients.
” ThePrint has reached Pfizer over email for comment on the report recommendations. This report will be updated if and when a response is received. Also Read: Cancer striking and claiming more lives among Indian women than men, shows ICMR study The researchers have underlined that nearly 70 percent of patients with lung cancers in India present with locally advanced and metastatic disease, with adenocarcinoma being the predominant histology.
With the development of precision oncology, the determination of targetable oncogenic drivers in NSCLC such as epidermal growth factor receptor (EGFR) mutations, or the anaplastic lymphoma kinase (ALK) and c-ros oncogene (ROS1) rearrangements has become important, they have also said. The prevalence of EGFR mutations and ALK rearrangements is approximately 30 percent and 10 percent respectively and similarly, the prevalence of ROS1 rearrangement is reported between 2.82 – 4.
1 percent. They also found that ALK inhibitors— anti-cancer drugs that target tumours with anaplastic lymphoma kinase (ALK), a protein that drives the growth of cancer cells —like crizotinib, ceritinib and lorlatinib have shown promise in treating patients with ALK-positive advanced or metastatic NSCLC. “However, treatment options are limited and crizotinib is a promising ALK inhibitor for ROS1-positive NSCLC.
Molecular testing and the targeted drugs are expensive and inaccessible to the majority of patients in low and middle income countries,” the report notes. In the absence of targeted therapy, treatment of patients with ALK- and ROS1-positive advanced/metastatic NSCLC—the latter being a subtype of lung cancer in which cancer cells have an abnormal fusion or mutation involving the ROS1 gene — is typically limited to cytotoxic chemotherapy regimens, which are more toxic and have greater side-effects leading to poor quality of life in patients. “In view of the above, economic evaluation becomes necessary to guide decision-makers about treatment choice, resource allocation and value for money,” the report reads.
Their analysis has shown that the treatment of patients with newly diagnosed ALK-positive advanced or metastatic NSCLC incurred a lifetime discounted cost of Rs 3.32 lakh, Rs 12.84 lakh and Rs 23.
37 lakh in the chemotherapy, crizotinib and ceritinib arms respectively. The mean quality-adjusted life year or QALYs—which is a measure of disease burden that considers both the quality and quantity of life lived and was considered the main health benefit in the evaluation—lived in each treatment arm were 1.20, 2.
21 and 3.34 months respectively. Similarly, patients with ROS1-positive NSCLC incurred a lifetime cost of Rs 3.
23 lakh and Rs 17.63 lakh for chemotherapy and crizotinib treatment arms respectively. The mean QALYs lived were 1.
16 and 2.73 months respectively. Some independent oncologists, too, pointed out that targeted cancer treatment offers a very potential and improved effectiveness and reduced side-effects compared to the traditional therapies by specifically targeting cancer cells while sparing the healthy cells.
“Targeted therapy gives a personalised treatment, because it is tailor-made to the specific characteristics of the patient’s cancer, leading to better outcome,” Dr Rathna Devi, senior consultant–radiation oncology at Chennai’s Apollo Cancer Centre, told ThePrint. (Edited by Gitanjali Das) Also Read: Cancer registry covers only 10% of Indians. Call to make it notifiable disease is gathering steam var ytflag = 0;var myListener = function() {document.
removeEventListener('mousemove', myListener, false);lazyloadmyframes();};document.addEventListener('mousemove', myListener, false);window.addEventListener('scroll', function() {if (ytflag == 0) {lazyloadmyframes();ytflag = 1;}});function lazyloadmyframes() {var ytv = document.
getElementsByClassName("klazyiframe");for (var i = 0; i < ytv.length; i++) {ytv[i].src = ytv[i].
getAttribute('data-src');}} Save my name, email, and website in this browser for the next time I comment. Δ document.getElementById( "ak_js_1" ).
setAttribute( "value", ( new Date() ).getTime() );.