ROME , Nov. 19, 2024 /PRNewswire/ -- A comprehensive study led by Professor Dr. Achille Gaspardone was presented at the 2024 TCT Congress which, demonstrated the exceptional efficacy of the NoblestitchTM suture-mediated Patent Foramen Ovale (PFO) closure system in preventing recurrent strokes among patients with PFO associated stroke.
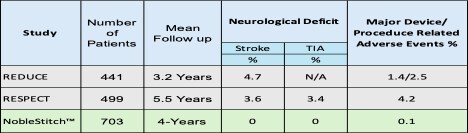
Involving 703 patients treated between 2016 and 2023, the study reported no recurrent strokes or transient ischemic attacks (TIAs) over an average follow-up of four years, underscoring NoblestitchTM as a highly effective solution for minimally invasive PFO closure. Key Findings Efficacy Outcome: Zero recurrent strokes or TIAs were observed across all 703 patients during the four-year follow-up period. Safety Profile: NoblestitchTM exhibited a robust safety profile with no suture-related complications.
Outcomes vs. Technical success: NoblestitchTM exhibited an 88% technical success at 12 month follow up. The sutured PFO configuration resulted in no strokes or TIA's independent of technical closure rates.
Dr. Gaspardone stated: "Suture mediated PFO closure is a long-term safe and effective procedure. The clinical outcomes of the suture mediated closure in our series are superior to the reported conventional umbrella closure device studies 1,2 for reduction of recurrent stroke and for reduction of device related complications including atrial fibrillation".
Dr. Anthony Nobles President of Nobles Medical Technologies II commented: "This landmark study showed no stroke or TIA's in 703 patients with 4-year mean follow up. In contrast, to published Gore Helix® and AmplatzerTM device studies which, reported stroke rates of 4.
7% and 3.6%, TIA rates of 3.4%, and device-related complication rates of 1.
4% and 3.4%, respectively. A Paradigm Shift: The "Suture First" Strategy Dr.
Gaspardone further commented: "This is the largest cohort of consecutive patients to definitively show the extraordinary efficacy and safety of the NobleStitchTM for PFO closure. With a 4-year mean follow up of zero strokes and no device related complications in 703 consecutive patients this affirms the stitch as the first choice for PFO closure". NoblestitchTM represents a transformative approach to PFO closure, with the "Suture First" technique showing clear advantages in safety and long-term efficacy, especially in patients with favorable anatomical profiles.
The use of the LASSO score further aids in predicting successful closure, ensuring high technical success rates. This study affirms that NoblestitchTM is a reliable, device-free option for PFO closure, setting a new benchmark in procedural safety and effectiveness for cryptogenic stroke patients. For more information, please contact: [email protected] or [email protected] Website: noblesmed2.
com About NoblestitchTM NoblestitchTM is a patented pioneering suture-mediated cardiovascular suturing technology developed to address structural heart defects in patients. It offers a minimally invasive, device-free approach designed to minimize adverse events and maximize long-term health outcomes. NobleStitch is Manufactured and distributed by HeartStitch, Inc.
The NobleStitch EL is FDA cleared in the USA and CE Marked in the EU for cardiovascular suturing and approved for PFO closure in the EU. 1 N Engl J Med. 2017;377:1022–1032.
doi: 10.1056/NEJMoa1610057 2 https://www.nejm.
org/doi/full/10.1056/NEJMc2033779 SOURCE HeartStitch® Inc..














