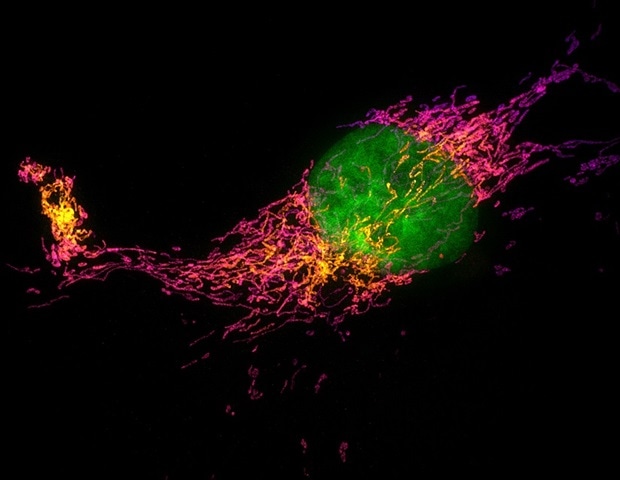
A team of researchers led by Anna-Karin Gustavsson at Rice University has developed an innovative imaging platform that promises to improve our understanding of cellular structures at the nanoscale. This platform, called soTILT3D for single-objective tilted light sheet with 3D point spread functions (PSFs), offers significant advancements in super-resolution microscopy, enabling fast and precise 3D imaging of multiple cellular structures while the extracellular environment can be controlled and flexibly adjusted. The research was recently published in Nature Communications .
Studying cells at the nanoscale provides insights into the intricate mechanisms that drive cellular behavior, enabling researchers to uncover details that are essential for understanding health and disease. These details can reveal how molecular interactions contribute to cellular functions, which is critical for advancing targeted therapies and understanding disease pathogenesis. While conventional fluorescence microscopy has been useful for studying cellular structures, it has been limited by the diffraction of light, restricting its ability to resolve features smaller than a few hundred nanometers.

Moreover, while single-molecule super-resolution microscopy has provided groundbreaking insights into biological structures at the nanoscale, existing techniques often suffer from high background fluorescence and slow imaging speeds, particularly when dealing with thick samples or complex cell aggregates. They also typically lack precise, adjustable control of the sample environment. The soTILT3D platform directly addresses these challenges.
By synergistically integrating an angled light sheet, a nanoprinted microfluidic system and advanced computational tools, soTILT3D significantly improves imaging precision and speed, allowing for clearer visualization of how different cellular structures interact at the nanoscale -; even in conventionally challenging samples. Key innovations The soTILT3D platform uses a single-objective tilted light sheet to selectively illuminate thin slices of a sample, effectively enhancing the contrast by reducing background fluorescence from out-of-focus areas, especially in thick biological samples such as mammalian cells. "The light sheet is formed using the same objective lens as used in the microscope for imaging, and it is fully steerable, dithered to remove shadowing artifacts that are common in light sheet microscopy and angled to enable imaging all the way down to the coverslip," said Gustavsson, assistant professor of chemistry at Rice and corresponding author of the study.
"This allows us to image entire samples from top to bottom with improved precision." The platform also incorporates a custom-designed microfluidic system with an embedded customizable metalized micromirror, which enables precise control over the extracellular environment and allows for rapid solution exchange, which is ideal for sequential multitarget imaging without color offsets while also allowing for reflection of the light sheet into the sample. "The design and geometry of the microfluidic chip and nanoprinted insert with the micromirror can be easily adapted for various samples and length scales, providing versatility in different experimental setups," said Nahima Saliba, co-first author of the paper alongside fellow graduate student Gabriella Gagliano, who is also associated with the Smalley-Curl Institute and the Applied Physics Graduate Program at Rice.
Additionally, soTILT3D leverages computational tools such as deep learning for analysis of higher fluorophore concentrations for improved imaging speed and algorithms for real-time drift correction, enabling stable, high-precision imaging over extended periods of time. The platform's PSF engineering enables 3D imaging of single molecules, while deep learning handles dense emitter conditions which conventional algorithms have trouble with, which significantly improves the acquisition speed." Nahima Saliba, Rice University Related Stories AI-powered ECG model predicts heart disease risk with precision New Alzheimer's guidelines focus on risk, not diagnosis, in healthy adults From womb to midlife: Prenatal immune disruptions reshape memory and cognitive aging SoTILT3D's microfluidic device also supports automated Exchange-PAINT imaging, allowing different targets to be visualized sequentially without the color offsets common in multicolor approaches when imaging in-depth at the nanoscale.
Groundbreaking results The soTILT3D platform has demonstrated remarkable improvements in imaging precision and speed. The platform's angled light sheet improves the signal-to-background ratio for cellular imaging by up to six times compared to traditional epi-illumination methods, improving contrast and enabling precise nanoscale localization. "This level of detail reveals intricate aspects of 3D cell architecture that have been traditionally difficult to observe with conventional approaches," said Gagliano.
In terms of speed, soTILT3D delivers a tenfold increase when combined with high emitter density and deep learning analysis, allowing researchers to capture detailed images of complex structures like the nuclear lamina, mitochondria and cell membrane proteins across entire cells in a fraction of the usual time. Additionally, the platform supports accurate whole-cell 3D multitarget imaging, capturing the distributions of multiple proteins within an entire cell and measuring nanoscale distances between them. Researchers can now visualize the spatial arrangement of closely situated proteins like nuclear lamina proteins lamin B1 and lamin A/C and lamina-associated protein 2 with remarkable precision and accuracy, offering new insights into protein organizations and their role in regulating cellular function.
Broad applications in biology and medicine The soTILT3D platform opens new possibilities for researchers across various fields. Its capability to image complex samples, including stem cell aggregates, extends its application beyond individual cells. The microfluidic system's biocompatibility makes it suitable for live-cell imaging, allowing scientists to study cellular responses to different stimuli in real time with reduced photo damage.
Its precisely controlled solution exchange feature also makes soTILT3D an ideal tool for testing how drug treatments affect cells in real time. "Our goal with soTILT3D was to create a flexible imaging tool that overcomes limitations of traditional super-resolution microscopy," said Gustavsson. "We hope these advancements will enhance studies in biology, biophysics and biomedicine, where intricate interactions at the nanoscale are key to understanding cellular function in health and pathogenesis.
" Rice University Saliba, N., et al . (2024) Whole-cell multi-target single-molecule super-resolution imaging in 3D with microfluidics and a single-objective tilted light sheet.
Nature Communications . doi.org/10.
1038/s41467-024-54609-z ..