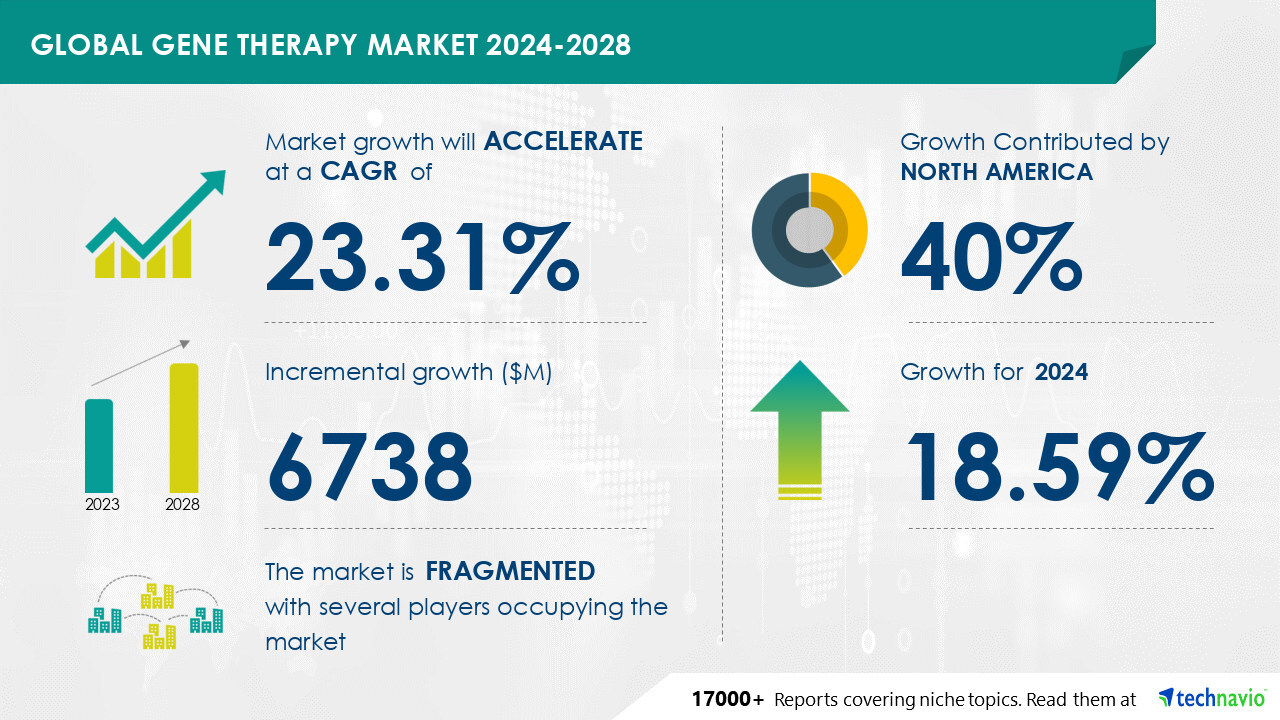
NEW YORK , Nov. 7, 2024 /PRNewswire/ -- Report on how AI is driving market transformation - The global gene therapy market size is estimated to grow by USD 6.74 billion from 2024-2028, according to Technavio.
The market is estimated to grow at a CAGR of 23.31% during the forecast period. Increase in special drug designations is driving market growth, with a trend towards growing research in gene therapy for CVDs and orphan diseases.
However, high treatment cost poses a challenge.Key market players include Abeona Therapeutics Inc, Adaptimmune Therapeutics plc, Adverum Biotechnologies Inc, Amgen Inc., Astellas Pharma Inc.
, Biogen Inc., bluebird bio Inc., Editas Medicine Inc.
, ElevateBio, F. Hoffmann La Roche Ltd., Generation Bio Co.
, Gilead Sciences Inc., Novartis AG, Orchard Therapeutics Plc, Poseida Therapeutics Inc., Sangamo Therapeutics Inc, Sibiono GeneTech Co.
Ltd., Syncona Ltd., uniQure NV, and Voyager Therapeutics Inc.
Key insights into market evolution with AI-powered analysis. Explore trends, segmentation, and growth drivers- View Free Sample PDF Market Driver The gene therapy market is experiencing significant growth due to the increasing prevalence of chronic diseases, particularly cardiovascular diseases (CVDs), and the need for effective and timely treatments. Major pharmaceutical companies are investing heavily in research and development (R&D) of novel gene therapies, particularly for diseases with a monogenic cause, such as many orphan diseases.
The aging population, with a high prevalence of CVDs in adults over 65 years, is driving the focus on developing gene therapies for coronary artery disease and heart failure. The delivery technology for these therapies, primarily using AAV vectors, is a significant area of R&D investment. Several gene therapies are expected to enter the market during the forecast period, contributing to the market's growth.
Gene therapy is a rapidly growing market with trends including oncolytic viral therapy, immunotherapy, and gene editing technologies. The gene delivery method and vector used are key considerations, with viral vectors like AAV being popular. The regulatory landscape is evolving, with reimbursements and commercialization strategies crucial for market success.
Several gene therapies have been marketed, such as Luxturna for vision loss and Zolgensma for spinal muscular atrophy. Mechanism of action varies, from replacing or repairing defective genes to triggering an immune response. Patent portfolio strength and emerging technologies are important for competitive advantage.
Clinical research initiatives in the genomics field are driving advanced targeted therapies. Traditional medicines are being complemented by gene therapy products. Preclinical testing, clinical trials, and regulatory approval are crucial steps in therapy development.
Neurology indications are a major target area, with temperature control and shelf life being important considerations for commercial success. Startups and big pharma players are collaborating to expand the clinical pipeline. Request Sample of our comprehensive report now to stay ahead in the AI-driven market evolution! Market Challenges Gene therapy is a revolutionary medical treatment with the potential to cure genetic disorders by replacing or repairing faulty genes.
However, the high cost of gene therapies, ranging from USD300,000 to USD1,200,000 per patient, is a significant challenge for the global gene therapy market. This cost is primarily due to the need for individualized treatment and intensive clinical trials. The process involves extracting the mutated gene from the patient, modifying it in the lab, and introducing the modified stem cells back into the patient using a viral vector.
The development of viral vectors and the technology to deliver the virus to patients also contributes to the high cost. For instance, Glybera, a gene therapy developed by uniQure for the treatment of lipoprotein lipase deficiency, costs around USD1,200,000 per patient. Despite being approved in Europe , uniQure failed to receive approval in the US due to lack of efficacy and high costs.
New technologies are emerging to reduce research costs, but the high costs associated with gene therapy are expected to remain a significant challenge, potentially impeding market growth during the forecast period. The gene therapy market is experiencing significant growth due to clinical research initiatives in neurology, oncology, and hepatology indications. Advanced targeted therapies, such as gene therapy products, are gaining popularity over traditional medicines.
The clinical pipeline is filled with promising gene therapy treatments undergoing preclinical testing and clinical trials. Regulatory approval is a key challenge, requiring extensive research and infrastructure investment. Neurology indications, like metachromatic leukodystrophy, are being addressed through gene replacement and gene editing modalities.
Viral vectors and non-viral vectors serve as molecular carriers for gene modification and gene editing. Shelf life, temperature control, and biological therapies pose additional challenges. Advanced technologies, including gene silencing, cell replacement, gene augmentation, and nanoparticles, are driving innovation.
Capital investments in gene therapy market are essential for disease indications, gene modification, genome sequencing, and manipulation technologies. Infrastructure in hospitals is crucial for the successful implementation of these advanced therapies. Discover how AI is revolutionizing market trends- Get your access now! Segment Overview This gene therapy market report extensively covers market segmentation by 1.
1 In vivo 1.2 Ex vivo 2.1 Oncology 2.
2 CNS 2.3 Ophthalmology 2.4 Rare diseases 2.
5 Others 3.1 North America 3.2 Europe 3.
3 Asia 3.4 Rest of World (ROW) 1.1 In vivo- In vivo gene therapy is a technique that delivers genetic material directly into target cells to modify their genetic makeup for therapeutic purposes.
Approved in vivo gene therapies include transporting functional copies of missing or faulty genes into target cells using viral vectors, such as adenovirus, adeno-associated virus (AAV), lentivirus, and retrovirus. AAV vectors, which contain no viral genes, are commonly used for long-term gene expression and reduce the likelihood of an immune response. Currently, three commercial in vivo gene therapies are available, and many more are in development.
In vivo gene therapy treats various medical conditions, including certain types of cancer and genetic and inherited disorders. The increasing use of in vivo gene therapy for these conditions will drive the growth of the global gene therapy market through the in vivo segment during the forecast period. AAV vectors, which can last the full life of a specific cell, reduce the number of therapy administrations and are frequently used in research requiring long-term expression.
Download a Sample of our comprehensive report today to discover how AI-driven innovations are reshaping competitive dynamics Research Analysis Gene therapy is an innovative approach in the healthcare industry that involves introducing genetic material into cells to treat or prevent diseases. Clinical research initiatives in the genomics field are driving the development of advanced targeted therapies using gene therapy products. These therapies offer a potential alternative to traditional medicines, particularly for disease indications in neurology and oncology.
The clinical pipeline for gene therapy, with preclinical testing and clinical trials underway for various indications. Regulatory approval is a crucial step in bringing these therapies to market, with viral vectors and non-viral vectors being used as molecular carriers. Gene therapy encompasses various modalities such as gene silencing, cell replacement, and gene augmentation.
Shelf life and temperature control are critical considerations for the effective delivery of these biological therapies. Gene therapy holds promise for treating conditions like metachromatic leukodystrophy through gene replacement and gene editing. Market Research Overview Gene therapy is a revolutionary medical approach that involves introducing genetic material into cells to treat or prevent diseases.
Clinical research initiatives in the genomics field are driving the development of advanced targeted therapies through the use of gene therapy products. Traditional medicines are being replaced with biological therapies, including gene therapy, which are undergoing preclinical testing and clinical trials for regulatory approval. Neurology indications, such as metachromatic leukodystrophy, are among the therapeutic areas being targeted with gene replacement and gene editing modalities.
Viral vectors, non-viral vectors, and nanoparticles are used as molecular carriers for gene delivery, with various vector types and delivery methods under investigation. The gene therapy market is expanding rapidly, with oncolytic viral therapy, immunotherapy, and gene editing therapies among the emerging technologies. The regulatory landscape, reimbursements, and commercialization strategies are crucial considerations for companies developing gene therapies.
Mechanisms of action include gene silencing, cell replacement, and gene augmentation, with a strong patent portfolio and infrastructure being essential for market success. Capital investments are being made in startup companies and big pharma players alike to advance therapy development platforms and clinical studies. Advanced technologies, such as genome sequencing manipulation technologies, molecular switches, and hybrid vector systems, are also playing a role in the gene therapy market.
Table of Contents: 1 Executive Summary 2 Market Landscape 3 Market Sizing 4 Historic Market Size 5 Five Forces Analysis 6 Market Segmentation Delivery Mode In Vivo Ex Vivo Therapy Area Oncology CNS Ophthalmology Rare Diseases Others Geography North America Europe Asia Rest Of World (ROW) 7 Customer Landscape 8 Geographic Landscape 9 Drivers, Challenges, and Trends 10 Company Landscape 11 Company Analysis 12 Appendix About Technavio Technavio is a leading global technology research and advisory company. Their research and analysis focuses on emerging market trends and provides actionable insights to help businesses identify market opportunities and develop effective strategies to optimize their market positions. With over 500 specialized analysts, Technavio's report library consists of more than 17,000 reports and counting, covering 800 technologies, spanning across 50 countries.
Their client base consists of enterprises of all sizes, including more than 100 Fortune 500 companies. This growing client base relies on Technavio's comprehensive coverage, extensive research, and actionable market insights to identify opportunities in existing and potential markets and assess their competitive positions within changing market scenarios. Contacts Technavio Research Jesse Maida Media & Marketing Executive US: +1 844 364 1100 UK: +44 203 893 3200 Email: [email protected] Website: www.
technavio.com/ SOURCE Technavio.














